[Press Release] Edgecare Inc. Obtains U.S. FDA Clearance for Ultrasound Bladder Scanner EdgeFlow UH10
Page Info.

Main Text
Edgecare Inc. Obtains U.S. FDA Clearance for Ultrasound Bladder Scanner EdgeFlow UH10
Seoul, South Korea – March 7, 2024 – Edgecare, a leader in ultrasound-based healthcare innovation, announced on March 7th that its bladder scanner, EdgeFlow UH10, has received clearance from the U.S. Food and Drug Administration (FDA). This clearance affirms the safety and effectiveness of Edgecare's product, representing a pivotal achievement for the company's expansion into the U.S. and global markets.
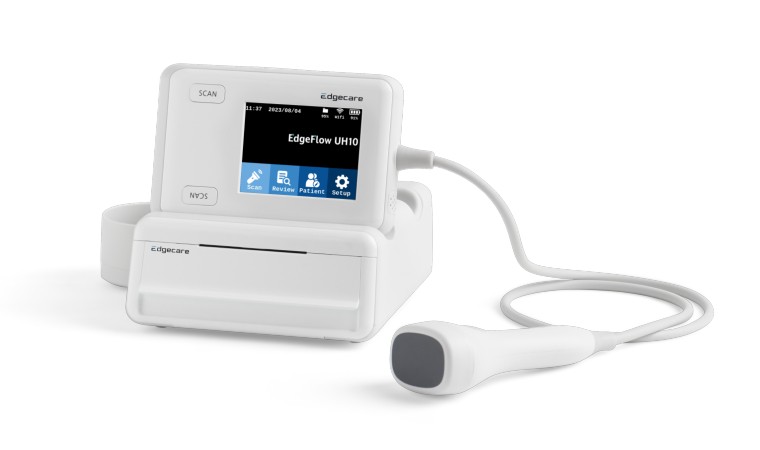
The EdgeFlow UH10 leverages high-resolution ultrasound imaging to non-invasively measure bladder volume, enhancing convenience in use and maintenance through an electronic probe. It incorporates LiveContour™ and MaxContour™, functionalities powered by artificial intelligence (AI) using deep learning technology. These advancements enable healthcare professionals, regardless of their experience level with ultrasound scanning, to easily locate the bladder and accurately assess its maximum volume. The device's compact design also ensures exceptional portability, allowing for unrestricted use across various settings.
Following this FDA clearance, Edgecare is poised to bolster its sales and marketing initiatives targeting U.S. medical institutions. The company aims to strengthen its market presence by offering solutions for the growing number of urinary health issues prevalent in aging populations. Furthermore, Edgecare plans to increase its global market share through strategic partnerships with worldwide medical device distributors.
Edgecare is set to participate in the American Urological Association (AUA 2024) in May. There, it will feature the EdgeFlow UH10 and actively promote its innovative product and technological excellence to urology professionals and hospital officials from around the globe.
Yangmo Yoo, CEO of Edgecare, expressed, "This FDA clearance lays the foundation for Edgecare to successfully operate not only within the U.S. but also in other international markets. We are committed to advancing monitoring solutions for escalating health concerns, contributing to the enhancement of human health and quality of life."

- Previous[Event] (3/24) 대한비뇨의학과의사회 춘계 학술대회 참가 24.03.15
- Next[Press Release] Edgecare Showcases EdgeFlow UH10 Bladder Scanner at Arab Health 2024 24.02.29